SCOTTSDALE DUI ATTORNEY DEFINITIVE GUIDE
Unique Issues and Special Information for Scottsdale, AZ DUI Cases
All You Need to Know About DUI in Scottsdale, AZ
KOPLOW LAW FIRM - EXPERIENCED scottsdale DUI Lawyer
Scottsdale DUI cases are different. Most people could not know this fact. Unless you have other DUI arrests to compare. The good news is you don’t have to get arrested somewhere else to learn the difference. Here are some important things only an experienced Scottsdale DUI attorney knows.
Ready to Fix This?
Essential Information About
Scottsdale, AZ DUI Cases
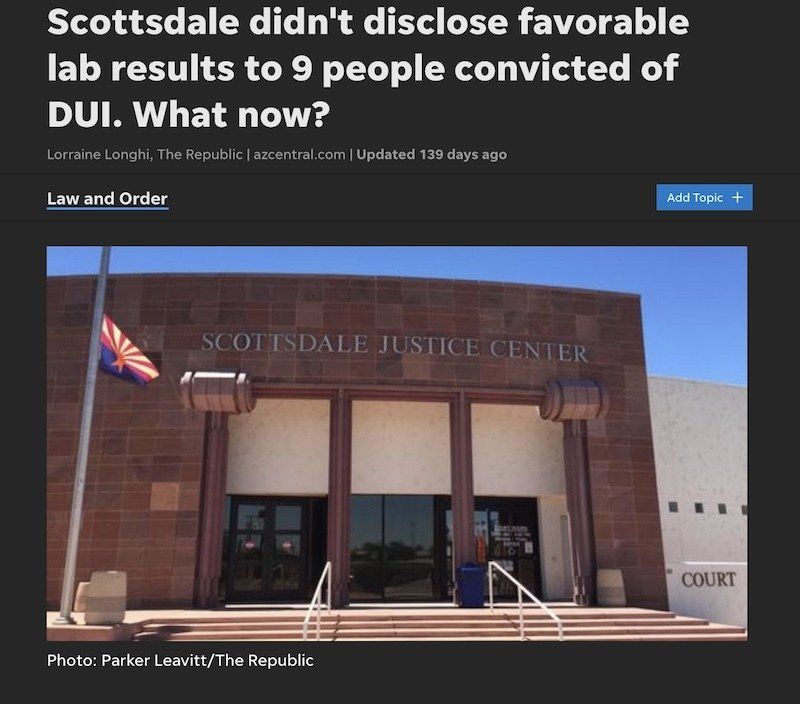
Scottsdale Crime Lab Scandals in DUI Cases
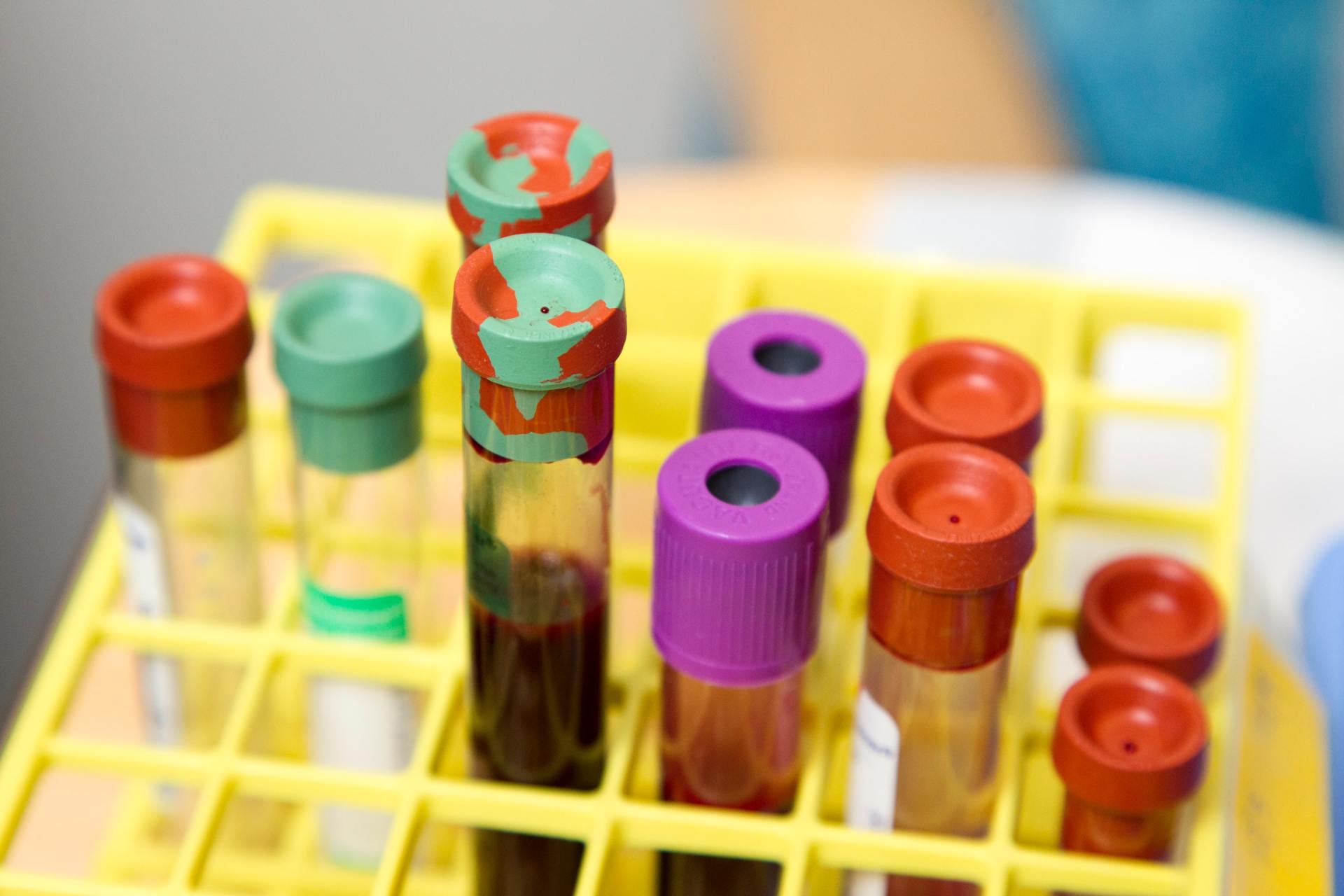
Scottsdale DUI cases almost always rely on the results of a chemical test(s). Thus, if you are arrested for a DUI in Scottsdale you need to know of any issues that cast doubt on the trustworthiness of the results of these chemical tests (i.e. blood and breath).
Software Errors
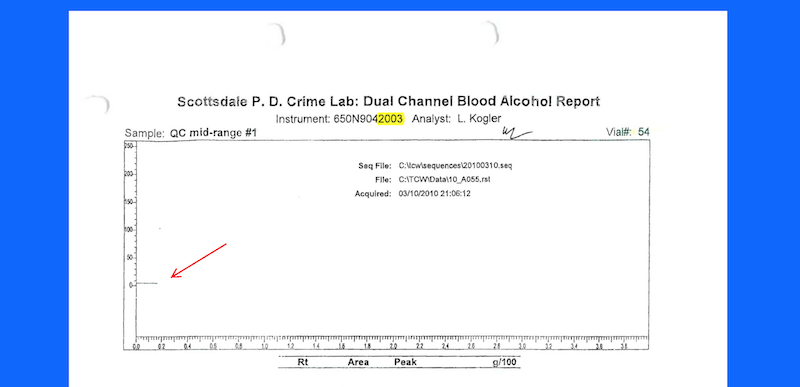
Between 2010-2016 the Scottsdale Crime Laboratory used instruments suffering from catastrophic software errors to test blood samples in Scottsdale AZ DUI cases. The kinds of errors that occurred included:
Myself, and a handful of other Scottsdale AZ DUI Lawyers, challenged the issue in a series of legal hearings. As these hearing went on systemic flaws in their testing process was revealed. Within the last few years we have still found unresolved software errors in the Scottsdale AZ crime laboratory.
In the News
- Arizona Supreme Court rules on Scottsdale DUI evidence
- Arizona DUIs in Question over Lab Methods
- Scottsdale DUIs in question over lab methods
- E-mails point to problems in Scottsdale crime lab
- Lab Results in DUI Cases In Arizona City Challenged
- $90K DUI machine goes unused in Scottsdale
Here is an article about a change in leadership at the Scottsdale AZ prosecutor’s office:
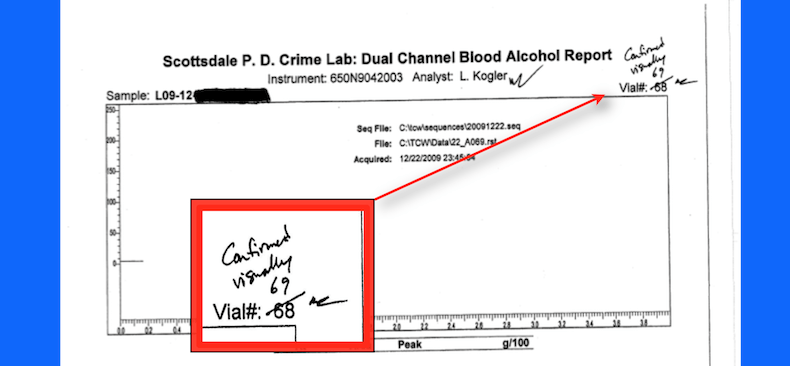
Blood Sample Tampering
In 2016, a blood sample was seized from a person accused of DUI. The blood sample is contained in a tube called a vacutainer. As is customary, a police officer collected two samples. Each sample in their own tube. The tubes of blood were sealed with red evidence tape.
A Scottsdale Crime Laboratory Analyst tested one of the blood samples for an alcohol concentration. This required her to open on of the blood tubes and break the red evidence tape. The second tube is intended to be in storage for the accused to test if they chose.
The analyst did not report any issues with her analysis. Her result claimed that the person’s sample contained an alcohol concentration above Arizona’s legal limit. The prosecution of the accused in Scottsdale City for DUI went forward based on her results.
She subsequently informed the laboratory that she was “planning on leaving the country” (you can’t make this stuff up if your tried). As a result, the lab states it retested several samples where the associated cases was set for trial.
When a member of the lab evaluated her work they essentially concluded that she attempted to cover a mistake. To do so she clandestinely opened the second tube and passed off a different result as her original test. If there was not a reason for someone to check her work (because of her plans to leave the country) it would have never been discovered.
Fire Destroys Blood Evidence
On July 13, 2019, an officer arrived at one of the Scottsdale Police Department’s Substations and noticed an odor of smoke. He discovered a refrigerator used to store DUI blood samples had been burned by fire. Several blood samples in DUI cases were also burned, damaged and destroyed.
How are Scottsdale DUI cases different from other DUI cases?
To start, the Scottsdale Police Department is very aggressive when it comes to DUI enforcement. Sometimes they are so aggressive that there is a danger of a rush to judgment. There is objective evidence demonstrating many people have been accused of DUI in Scottsdale and it was subsequently determined they were innocent.
The Scottsdale Police Department is one of the few law enforcement agencies that require a person suspected of DUI to perform both an intoxilyzer breath test and also provide a blood sample.
More news about Scottsdale City Prosecutor’s Office: Fired Scottsdale Prosecutor Sues City
How many DUI cases does the Scottsdale City Court handle each year?
Scottsdale police make a significant amount of DUI arrest each year. To give you an idea of the volume of DUI cases that are prosecuted in this court let’s look at 2018. According to the Scottsdale City Court’s 2018 Annual Report, 14,763 criminal matters filed: 434 were DUI cases.
Does the Scottsdale City Court make a profit?
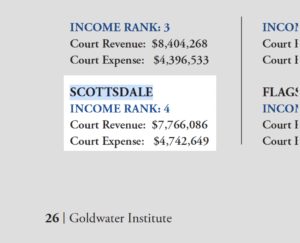
Accordingly to a 2016 study by the Goldwater Institute, not only does court have a net revenue, it’s actually one of the top-earning courts in Arizona. The Scottsdale City Court’s Revenue was: $7,766,086, but the court’s expenses were a mere $4,742,649 in comparison. As a result, the court had a net profit of approximately $3,000,000.00.
Does the Scottsdale Police Department have DUI quotas?
They will tell you no, but…
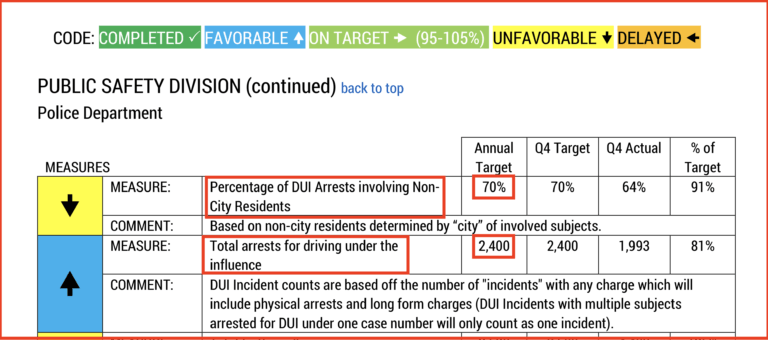
I discovered this by chance during a jury trial. Instead of using the word “quota” they call it a SMART Goal. As you can see from the above screenshot taken from a Scottsdale City Counsel Quarterly Report, the Scottsdale Police Department has a SMART goal 2400 DUI arrest per year.
They also have a goal to have 70% of those arrested to be of non-Scottsdale residents. Why would arresting more people, who don’t live in Scottsdale but commit the crime of DUI there, the make the city safer? (I live in Scottsdale and this statistic definitely does not make me feel safer).
Why is my DUI in Scottsdale City Court and not a Justice Court?
Most DUI arrests in Scottsdale are processed and prosecuted in the Scottsdale City Court. The deciding factor that determines what court your case will be sent is which police department makes the arrest.
When an officer makes an arrest they have discretion to submit to the case to any court that have jurisdiction. In scottsdale there are two possible court. The Scottsdale City court or the West Mesa Justice Court (the name is misleading).
Does the Scottsdale Court permit an out-of-state resident to resolve a DUI without returning to Arizona?
It is legally possible for a person arrested for DUI who was merely visiting Scottsdale to resolve their case without returning to Arizona. However, this is a very fact-specific issue.
Why didn’t the officer read me my Miranda rights until the investigation was almost over?
Scottsdale is one of the only police departments in Arizona that will wait until the entire DUI investigation is almost completed – to inform you of your legal rights. Their standard practice is to inform you of your right to consult with a lawyer after obtaining your consent to a chemical test.
Does the Scottsdale City Court have a way to look up information on a DUI case up online?
Yes. However, a word of caution – the court’s online system is not always correct and there are often delays between when the court (or law enforcement) makes a decision and when it posted online. Here is a link Scottsdale City Court’s CASE SEARCH.
Do Scottsdale police officers have body cameras that record video?
Yes. In 2017, the Scottsdale Police Department announced they would be switching to the Axon Flex 2 body camera.
According to Scottsdale Police Department Policy officer are required to activate their “on-body camera” (OBC) to record all citizen contacts when performing their official duties. However, the videos they provide often are muted or edited. If a criminal defense attorney wants to see if this has occurred then they might be able to obtain metadata from the recording software.
The Scottsdale Police Department has started to acknowledge some of the problems that have arisen since officers have started using on-body cameras. Here are the results of a June 2018 internal Police Body Camera Audit: AUDIT REPORT NO. 1808
Scottsdale Jury Duty
The Scottsdale City Court contracts with Maricopa County Superior Court to handle the process of sending jury duty summonses to potential jurors by mail. Effective June 30, 2020, the Scottsdale City Counsel will extend their contract with the Superior court another two-years.
Once a person receives a summons for jury duty and appears, the process of selecting which jurors in the “pool” will actually sit to decide a case is called voir dire. During this process the prosecutor and the criminal defense attorney will select the people that will actually hear the case.
Court Contact Information
Wondering "How do I get to the Scottsdale City Court?" Or maybe, "How to contact the Scottsdale court?"
Address: 3700 N 75th St, Scottsdale, AZ 85251
Phone: (480) 312-2442
Fax: 480-312-2764
Email: court@scottsdaleaz.gov
Website:
https://www.scottsdaleaz.gov
Scottsdale Police Officers on the Brady List
The United States Supreme Court, in the case of Brady v. Maryland, held that the government must disclose to the defense all evidence that is favorable or may exonerate a defendant. As a result, prosecution officers have created so called “Brady Lists” identifying officers that have demonstrated dishonesty through their conduct. Under Brady, the government must disclose exculpatory evidence to a criminal defense lawyer even without a request.
"Lawrence represents very high-profile clients who greatly depend on a good outcome, and this guy will deliver.
This is a prosecutor's worst nightmare, and it should be that way if you need an attorney."
- David E.
Real Client's Husband, Phoenix, AZ
For more information about our Scottsdale DUI lawyer, call our office at (602)-494-3444 or visit our contact page.
What Real People Are Saying
That is, DUI cases actually dismissed. Verifiable not guilty verdicts. Blood alcohol evidence truly suppressed as witnessed by judges, other lawyers, and newspapers.
Real Client's Husband, Phoenix, AZ
"Lawrence represents very high-profile clients who greatly depend on a good outcome, and this guy will deliver.
This is a prosecutors' worse nightmare, and it should be that way if you need an attorney."
- David E.
REAL CLIENT, PHOENIX, AZ
It was miracle!... A lot of people don't really understand the benefit of having an attorney who used to be a prosecutor. They know all the little tricks and scare tactics the state has as opposed to just hiring an attorney who is a little fish in a big pond."
- Joe C.
Contact Our Scottsdale DUI Lawyer Today!
For more information, call our office at (602) 494-3444 or fill out the form and we will get back to you.
Contact Us
We will get back to you as soon as possible.
Please try again later.
Legal Coffee Blog | Arizona DUI



DUI Library
The best DUI defense stuff that only a few know and none want to share. A one of a kind annotated resource for lawyers, people accused, or anyone who wants to see what’s going on in our justice system with DUI cases…and how to fix it.

Contact Information
Office Hours
- Mon - Fri
- -
- Sat - Sun
- Closed
What Happens After You Reach Out
A team member will begin reviewing your case.
We will contact you to ask questions and go over your options.
We will determine, together with you, what makes sense for the next step for you and your family to take.
Ready to Fix This?
Contact Us
We will get back to you as soon as possible.
Please try again later.
OUR SERVICES
QUICK LINKS
CONTACT US